Synthesis of Cadmium Sulfide Nanoparticles
The procedure shown here was adapted by Paul Hansen and George Lisensky from Kurt Winkelmann, Thomas Noviello, and Steven Brooks, "Preparation of CdS Nanoparticles by First-Year Undergraduates," J. Chem. Educ. (2007) 84, 709-710, which was based on M. L. Curri, A. Agostiano, L. Manna, M. D. Monica, M. Catalano, L. Chiavarone, V. Spagnolo and M. Lugarà, J. Phys. Chem. B, (2000) 104, 8391-8397.
Hexadecyltrimethylammonium bromide has a long hydrophobic chain and a polar head group.
The molecule does not dissolve well in either aqueous or organic solvents. In an organic solvent containing a small amount of water the hexadecyltrimethylammonium bromide traps the aqueous portion in a micelle sphere with the polar heads facing in and the non-polar tails facing out. The relative amount of pentanol cosurfactant controls the size of the micelle.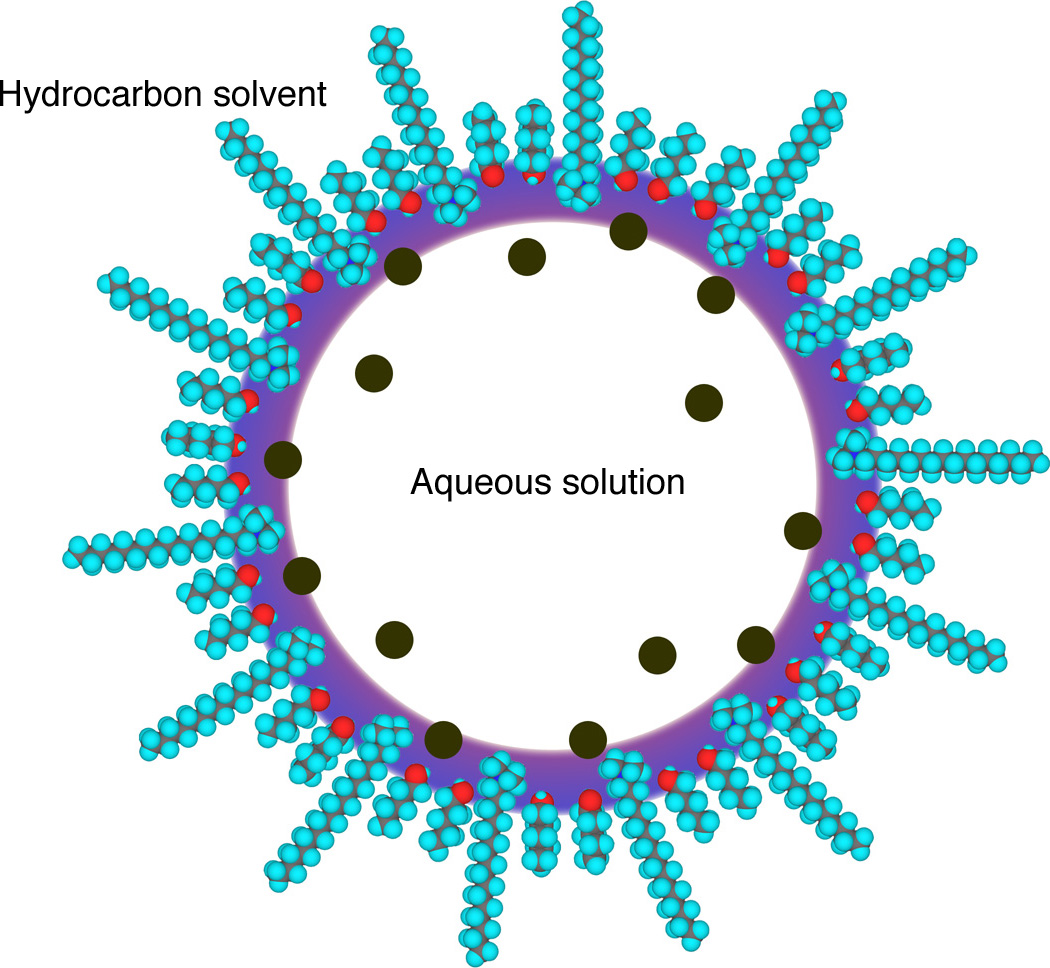 A water-in-oil microemulsion droplet. This static picture
A water-in-oil microemulsion droplet. This static picturedoes not properly convey "the dynamic reality of the aggregates."
Figure based on J. Phys. Chem. 100, 3190-3198 (1996).
Mixing hexadecyltrimethylammonium bromide pentanol micelles of CdCl2 with similar micelles containing Na2S produces nanoparticle CdS since the aqueous solution serves as a nanoreactor and the particles cannot grow bigger than the micelle. The pentanol also acts as a capping agent to stabilize the CdS particles. The formation of CdS nanoparticles can be detected by spectroscopy since quantum size effects make the visible absorption spectra different than that of bulk CdS.
| Procedure | Wear eye protection |
Chemical gloves recommended |
Test the reagents by adding a drop of aqueous Cd+2 to a drop of aqueous S-2. A yellow color should appear if the Na2S solution is good. If the mixture remains clear, remake the Na2S solution. In a plastic cuvet, add an equal amount of aqueous 0.012 M Cd+2 and aqueous 0.012 M S-2. Record your observations and immediately obtain the visible absorption spectrum (before the solution becomes too opaque.) Discard the solution in an appropriate waste container. Add 0.20 g hexadecyltrimethylammonium bromide to a test tube. Add a stir bar. Clamp over a magnetic stirrer. Add 4.0 mL heptane and 1.0 mL pentanol to the hexadecyltrimethylammonium bromide. Stir to give a suspension. Transfer half the suspension to a second tube. Stir both solutions to maintain the suspension. To one test tube, add 0.1 mL (about 3 drops) of 0.012 M CdCl2. Stop adding when the solution clears as hexadecyltrimethylammonium bromide micelles containing CdCl2 form.
To the second test tube, add 0.1 mL (about 3 drops) of 0.012 M Na2S. Stop adding when the solution clears as hexadecyltrimethylammonium bromide micelles containing Na2S form. Join the two solutions by rapidly pouring one into another. Stir. Record the visible absorption spectrum in a glass cuvet. (Heptane will dissolve plastic cuvets.) Discard the solution in an appropriate waste container when finished.
Calculations
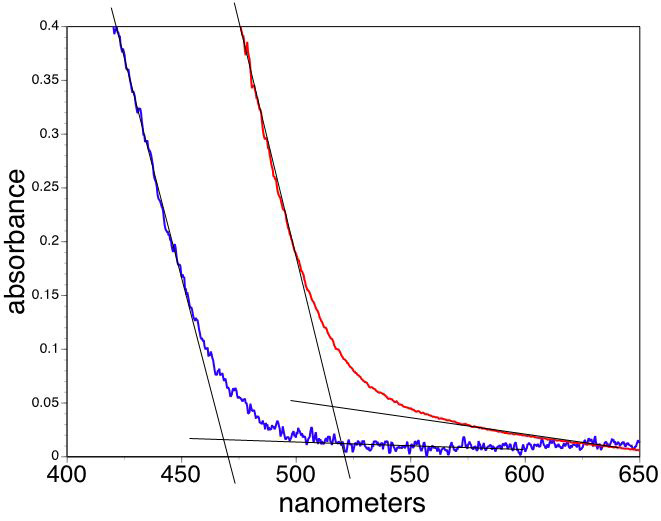
Extrapolate the linear portions of the lowest energy absorbance as a function of wavelength to find the band edge wavelength for your sample.
(One option is to use the band edge tab on the Beers Law template. Change the wavelength values in the colored shaded boxes to choose the ends of the linear portions of your absorbance graph. The program will least squares fit the interval and show the intersection of the two lines in the table. The other option is to transfer the wavelength and absorption data to the band edge spreadsheet and do the same analysis.)
Convert the band edge wavelengths to band gap energies, Egnano and Egbulk.
Eg = h c / λ
h = 6.626x10-34 J s
c = 2.998x108 m/s
e = 1.602x10-19 C
ε0 = 8.854x10-12 C2/N/m2
m0 = 9.110x10-31 kg
CdS
λbulk = 512 nm
ε = 5.7
me* = 0.19
mh* = 0.80
CdSe
λbulk = 709 nm
ε = 10.6
me* = 0.13
mh* = 0.45
ZnO
λbulk = 365 nm
ε = 8.66
me* = 0.24
mh* = 0.59
CuInS2
λbulk = 810 nm
ε = 11
me* = 0.16
mh* = 1.3
ZnS
λbulk = 340 nm
ε = 5.1
me* = 0.49
mh* = 0.28
PbS
λbulk = 3400 nm
ε = 17.2
me* = 0.25
mh* = 0.25
The effective mass model suggests
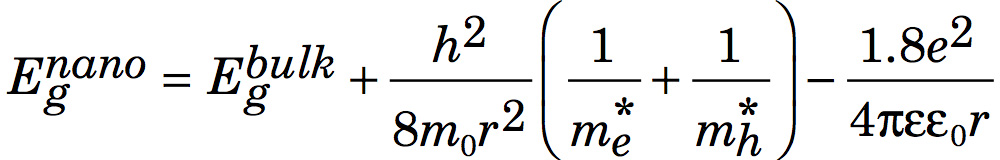
where r is the radius of the nanoparticle. The second term is the particle-in-a-box confinement energy for an electron-hole pair in a spherical quantum dot
and the third term is the Coulomb attraction between an electron and hole modified by the screening of charges by the crystal.
After multiplying by r2, rearranging, and using the quadratic formula to find r,
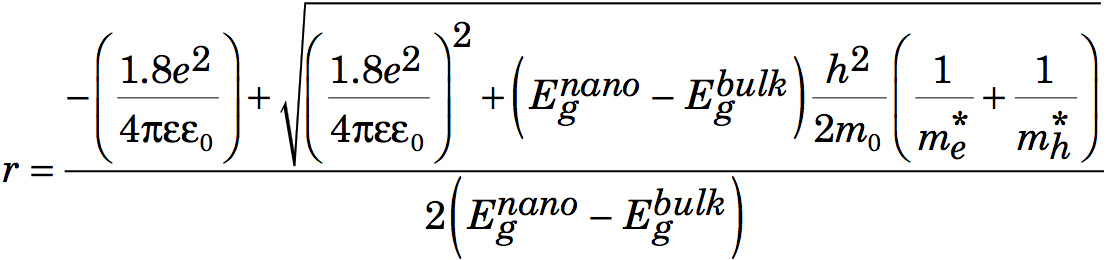
Conclusions
- Is the initial product from mixing aqueous cadmium ion with aqueous sulfide ion nanosize? Why do you need to take the spectrum quickly?
- If CTAB plus heptane plus pentanol results in a clear solution, what was wrong with the procedure?
- Is the band gap energy of the CdS nanoparticles larger or smaller than that of bulk CdS? Does this make sense?
- What is the diameter for your CdS nanoparticles?
Materials
CAUTION: Avoid physical contact with cadmium chloride and cadmium
sulfide as both are carcinogens.
- Stock Solutions for hundreds of batches
- 0.012 M CdCl2: Dissolve 0.110 g in 50 mL distilled water. This solution keeps for months.
- 0.012 M Na2S.9H2O: Dissolve 0.144 g in 50 mL distilled water. This solution does not keep well.
- Hexadecyltrimethylammonium bromide (Aldrich H9151) or Cetyltrimethylammonium bromide (alternate name CTAB)
- Heptane
- Pentanol
- Two test tubes, ring stand, test tube clamps
- Plastic dropper
- Two 1/4" magnetic stir bars, magnetic stirrer
- Glass cuvet and visible absorption spectrometer
Equipment
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified February 6, 2020.