Superhydrophobic Surfaces
(Electroless Deposition with Alkanethiol Treatment)
The electroless deposition idea for superhydrophobic surfaces is based on a paper by Iain A. Larmour, Steven E. J. Bell, and Graham C. Saunders, "Remarkably Simple Fabrication of Superhydrophobic Surfaces Using Electroless Galvanic Deposition," Angew. Chem. 2007, 119, 1740 –1742 and two papers by Feng Guo, Xunjia Su, Genliang Hou and Ping Li, "Bioinspired fabrication of stable and robust superhydrophobic steel surface with hierarchical flowerlike structure," Colloids and Surfaces A: Physicochem. Eng. Aspects 401 (2012) 61-67 and "Superhydrophobic silver surface with dendrites structure on steel substrate by a facile electroless galvanic deposition process," Applied Surface Science 258 (2012) 4906-4910 which use a different redox reaction and a different coating.
Begin by using a redox reaction to deposit copper nanoparticles on zinc, followed by treating part of the copper with octadecanethiol to give a metal thiolate. Then using drops of water as a probe and the principle of "like attracts like" classify which surfaces are like water.
| Procedure | Wear eye protection |
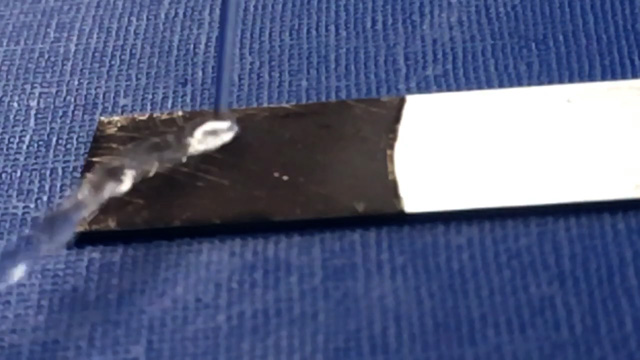
If you examined the surface by Scanning Electron Microscopy (SEM) you would see copper nanoparticles. Quarter-fill a 10 ml beaker with 0.004 M octadecanethiol in ethanol in order to dip half the dry copper coating for 60 seconds. Try not to move the metal so that the interface stays well defined. What do you observe? Remove the metal strip and wait for the ethanol to evaporate. You will then have three surfaces: the orignal zinc, copper on zinc, and octadecanethiol on copper on zinc.
Data
Test with small drops water to compare coated and uncoated regions. Do the water drops spread out or bead up?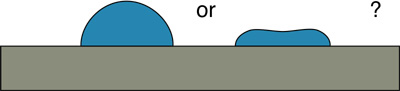
 Click image for larger view
Click image for larger view4µL water drop
- Make a sketch or include a photo of your final coating step that identifies the different surfaces you prepared.
- What happens when small drops of water are added to each of the surfaces?
- What happens if you drag a drop of water from the alkanethiol coating to the copper coating?
- Can you make a virtual wall by running water down the metal to the treated surface?
- Classify the Zn, the Cu nanoparticles, and the alkanethiol coated surface as like or unlike the water drop probe.
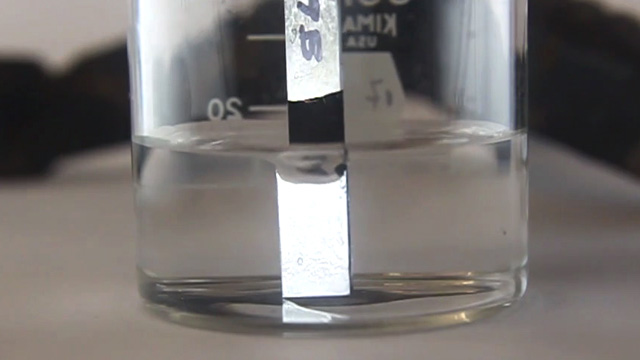
- What does the strip look like under water? Does the rotation angle change the appearance of the strip in water? If so, which of the surfaces change in appearance?
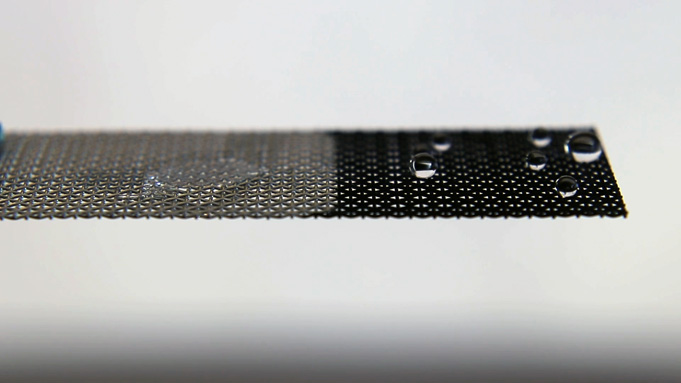
- Does the coating affect how water passes through a screen? If you did not have time to do this, make a prediction.
Scanning Electron Microscopy (SEM) (optional extension)
 Click image for larger view
Click image for larger viewSEM of Cu coating
Obtain a 10 mL beaker that is half-full of 0.080 M AgNO3 solution. Dip part of the strip in the solution for 20 seconds. What do you observe? What chemical reaction occurs?
Gold (optional extension)
Obtain a 10 mL beaker that is half-full of 0.010 M HAuCl4 solution. Dip part of the strip in the solution for 2-3 minutes until no new bubbles form. Agitate to remove bubbles for a more even coating. What do you observe? What chemical reaction occurs?
- 10 mL beakers
- Galvanized steel sheet (26 gauge, 12" x 18"), Ace Hardware 5080122, cut into 1 cm x 5 cm strips
- Galvanized Steel Wire Cloth 24 x 24 Mesh Size, 0.028" Opening Size, 1 Foot x 1 Foot, McMaster Carr 9220T92, cut into 1.5 cm x 5 cm strips
- Grade #0 steel wool
- 0.080 M CuSO4 with .080 M NaCl. Dissolve 10.2 g CuSO4.5H2O and 2.35 g NaCl in 500 mL water.
- CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2SH
0.004 M Octadecanethiol in 100% ethanol. Dissolve 0.12 g in 100 mL 100% ethanol. - Water for rinsing
Materials (for optional extensions)
- 0.080 M AgNO3. Dissolve 0.34 g AgNO3 in 25 mL water.
- 0.010 M HAuCl4. Dissolve 1.00g HAuCl4.3H2O in 250 mL water.
- Contact angle goniometer (optional)
- Electron microscope (optional)