Ph = -C6H5, Cy = -C6H11,
Me = -CH3, iPr = -CH(CH3)2, tBu = -C(CH3)3,
py = C5H5N, bipy =
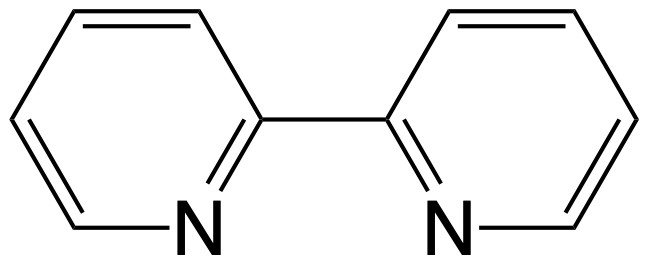 ,
,en = NH2CH2CH2NH2, acac = CH3COCHCOCH3–,
dien = NH2CH2CH2NHCH2CH2NH2, tacn =
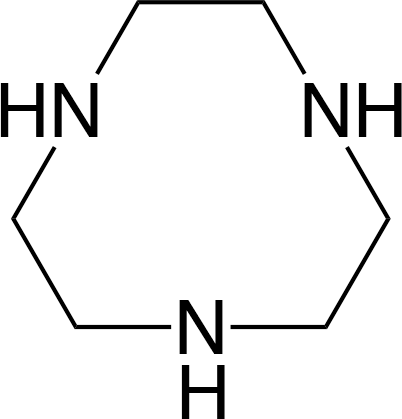
Counter ions not shown.
See original references for the crystal structures.
Sets in { } count as one problem.