The Petal Effect
Procedure adapted by Sue Whitsett from L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia, and L. Jiang, Langmuir, 24, 4114-4119 (2008). See also M. Nosonovsky and B. Bhushan, "Lotus versus Rose: Biomimemtic Surface Effects," in Green Tribology, Green Energy and Conservation, DOI: 10.1007/978-3-642-23681-5_2, Springer-Verlag (2012)
Micro and nanostructures on some flower petals provide a sufficient roughness to exhibit superhydrophobicity and a high adhesive force with water. A small water droplet on the surface of the petal appears spherical in shape, but will not roll off (Cassie impregnating wetting state).
| Procedure | Wear eye protection |
Chemical gloves recommended for PDMS |
Negative Image
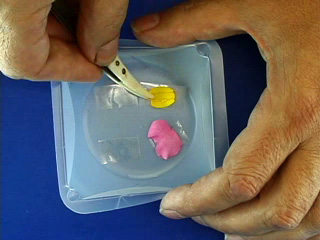
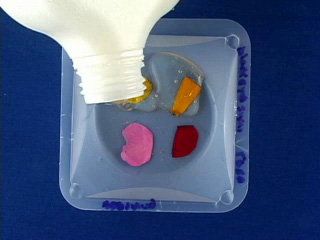
Place double sticky tape in a weighing boat. Add flower petals without touching the center portion of the petal. Pour about 5 mL of polyvinyl alcohol (PVA) solution into each weigh boat. (HINT: Leave part of the petal not covered with PVA for easier peeling later.) To evaporate water, let sit in a fume hood for a day or on a benchtop for a week. Positive Image
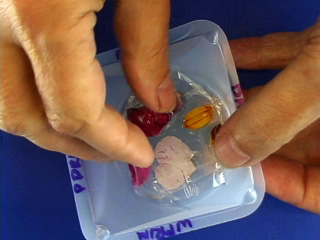
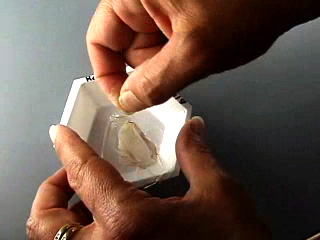
The dried PVA is hard. Remove the PVA from the weighing boat. Remove the double-sided tape. Remove the flower petal. This leaves a negative cast of the original petal. The imprint will be on the dull side of the polymer. Tape may be useful for removing the flower petal. Put double sticky tape on the bottom of a 5 ounce bathroom cup. Place the PVA mold shiny side down onto the tape. Cover with PDMS components. Air dry for about 3 days to cure (or place in an oven at 70° for 1 hour or 90°C for 30 minutes.) Remove the PDMS from the PVA. This gives a positive cast of the original petal. Measurements
Test the surface with 4µL drops of water. Take a photograph that shows a 4 µL drop sitting on a flat surface so you can measure contact angle of the water drop with the surface. What happens when you turn the cast upside down? Take another photograph for measuring. Slice a small section of the PDMS and mount on double stick carbon tape. Sputter coat with gold and obtain the SEM image. What are the sizes of the surface features? Questions
- Which flower did you test?
- Measure a photograph that shows the contact angle of a 4 µL water drop with your surface. Include your photograph.
What is your measured value of the contact angle when the drop is on the surface?
(ImageJ is useful for measuring angles. Import a photo. Select the angle tool. Double-click on the surface away from the water drop. Click where the water drop meets the surface. Click again at the drop above the surface to mark the angle. Drag the boxes at the ends of the line segments to adjust and see the value of the angle.)
Does the angle change when you turn the cast upside down? Include your photograph.
What is your measured value of the contact angle when the drop is under the surface? - Differences between flowers could be due to surface chemistry or surface physics. How does making a cast help answer whether differences depend on chemistry or physics?
- What are the sizes of the surface features as measured by SEM?
(ImageJ is useful for measuring lengths. Use the straight line tool to drag the length of the scale bar and menu Analyze/Set Scale to tell the program the actual length of the scale bar. You can then use the straight line tool to measure lengths in real units.) - Identify two practical uses that could be developed based on this experiment. For ideas, see the original paper Citing Articles (bottom left after you click the link).
- PVA preparation (below): Polyvinyl alcohol (Aldrich 348406, MW 13,000-23,000, 98% hydrolyzed), 500 mL beaker, hot plate stirrer and large stir bar.
- Weighing boats (100 mL)
- Double sticky tape
- Flower petals
- PDMS preparation (below): PDMS base and curing agent (Dow Corning Sylgard Elastomer 184 Kit, available from Ellsworth Adhesive), aluminum foil, disposable gloves, large weighing boat, plastic spoon, dropper, stir sticks
- A micropipet that can deliver 4 µL
Prepare 15 wt% polyvinyl alcohol for about 50 batches
For a smaller batch: stir 68 mL of water on a hot plate, set to medium heat, add 12 g of polyvinyl alcohol,
and continue to stir and heat until all of the polyvinyl alcohol has dissolved. Let cool 30 minutes before use.
Prepare 10% polydimethylsiloxane
Dispensing the viscous polydimethylsilane (PDMS) components can be messy. Cover the work surface and the balance with aluminum foil. Wear gloves.For one sample, add 4.00 g of Sylgard polymer base to a large weighing boat using a disposable plastic spoon. Add 0.40 g of curing agent using a disposable dropper to make a 10% mixture. Thorough mixing of the PDMS components is essential for good curing. Improper mixing can result in a polymer that is a sticky mess. On the order of 100 strokes with a stir stick are needed to mix the polymer components so that they will yield an adequately cured sample. PDMS is cured by an organometallic crosslinking reaction. The mixture should be used within one hour. For a larger batch, add 40.0 g Sylgard polymer base and 4.0 g curing agent and mix thoroughly. The mixture should be used within one hour.
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified November 9, 2016 .