Preparation of Zeolite ZSM5 and Catalysis of Xylene Isomerization
This experiment is modified by G. Lisensky and I. Blitz from the synthesis by L. D. Rollman and E. W. Valyocsik, “Zeolite Molecular Sieves,” Inorganic Syntheses, 22, 61-68 (1983) and the catalytic reaction by S. Whittingham, http://chemiris.chem.binghamton.edu/chem445/zsm5/zsm5.htm.
Zeolites are three-dimensional, crystalline networks of AlO4- and SiO4 tetrahedra. Their crystallization is often a nucleation-controlled process from a gel and the structure that crystallizes depends on the cations present. ZSM-5, Zeolite Socony Mobil #5, is a catalyst first made by Argauer and Landolt in 1972 (U. S. Patent 3,702,886). It is a medium pore zeolite with channels defined by ten-membered rings. The synthesis involves three different solutions. The first solution is the source of alumina, sodium ions, and hydroxide ions; in the presence of excess base the alumina will form soluble Al(OH)4- ions. The second solution has the tetrapropylammonium cation that acts as a templating agent. The third solution is the source of silica, one of the basic building blocks for the framework structure of a zeolite. Mixing the three solutions produces supersaturated tetrapropylammonium ZSM-5, which can be heated to recrystallize and produce a solid. In subsequent steps the organic templating agent is removed and the zeolite converted to its acid form.
“A multitude of commercial processes have been developed that exploit size exclusion and selective molecular diffusivity properties based upon the nanosize pore and channel structure of zeolites… The demand for two C8 isomers, paraxylene and orthoxylene, is much greater than that for the C8 isomers metaxylene and ethylbenzene. H-ZSM5 with its 0.6 nm pore size has the ability to isomerize xylenes with little cracking of the feedstock. A second crucial property is that paraxylene has a much higher diffusivity in H-ZSM5 than do the other xylene isomers. This means that the paraxylene molecules can more easily diffuse out of the zeolite crystal, whereas the ortho and metaxylene isomers are effectively trapped within the pores.” -Vision for Nanotechnology R&D in the Next Decade, National Science and Technology Council IWGN Workshop Report, Section 9.7.4 Nanoscale Catalysts (1999).
| Procedure | Wear eye protection |
Thermal gloves recommended |
Carry out all steps in polypropylene containers to prevent contact
of the strongly basic solutions with glass. Use Teflon coated stir bars for mixing. |
Obtain the X-ray powder diffraction spectrum of Na-ZSM-5 by scanning from 5 to 28 degrees. Compare with the expected peaks at 11.1 (s), 10.0 (s), 3.85 (vs) and 3.71(s) Å.
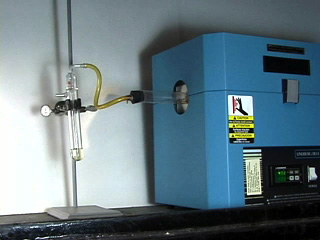
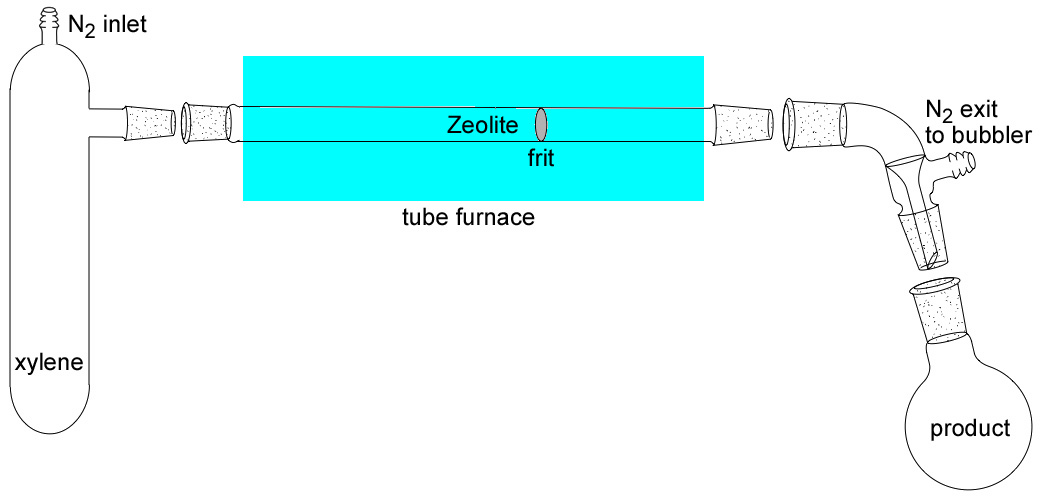
Place a known mass of H-ZSM-5 catalyst in the fritted adapter, followed by glass wool. (Do not use too much glass wool or pack the sample too tightly or the xylene will be unable to pass through!) Place 15 mL of o-xylene in the reflux flask. Flow nitrogen gas through apparatus (approximately 6-10 psi is required) at a low to moderate flow rate as indicated by the bubbler. Heat the tube furnace to 425°C and heat the xylene to its boiling temperature (140°C). Nitrogen acts as a carrier gas to transport the hot o-xylene vapor through the catalyst. Place the collection flask in an ice bath to ensure condensation of the xylenes.
Obtain a 1H NMR spectrum of both the initial o-xylene and the product. Use integration to determine the extent of conversion to p-xylene.
- Aluminum oxide, activated, neutral, Brockman I, 150 mesh (Aldrich 199974)
- NaOH
- LUDOX HS-30 colloidal silica, 30 wt% SiO2 (Aldrich 420824)
- Tetrapropylammonium bromide (Aldrich 225568)
- Ammonium sulfate
- Barium chloride
- acetone
- nitrogen
- o-xylene
- 100 mL weighing boats
- 1" magnetic stir bar and stirrer
- 125 mL wide mouth polypropylene bottles (VWR 16126-128)
- pH paper
- 95 degree C oven
- suction filtration apparatus
- alundum boat
- 500 degree C tube furnace
- 100 mL beaker
- test tubes
- catalytic reactor
- NMR spectrometer
Equipment
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .