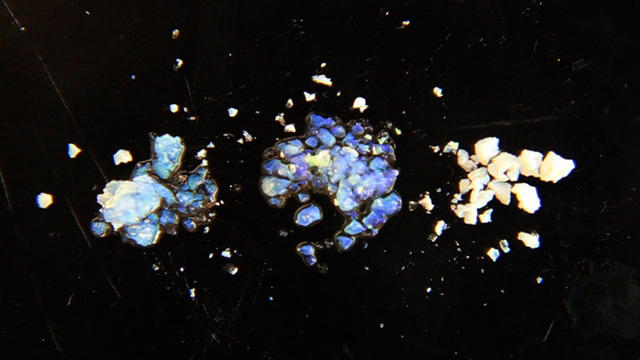
Synthesis of Inverse Opal Photonic Crystals
Procedure modified by George Lisensky, Jacob Horger and Jiaqi Luo, Beloit College, from the Inverse Opal Photonic Crystals Laboratory Guide by R. Schroden and N. Balakrishnan, University of Minnesota MRSEC, 2001.
A sol-gel synthesis using tetraethylorthosilicate and close-packed polymethylmethacrylate spheres as a template yields a dimensionally ordered porous silica solid.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood recommended |
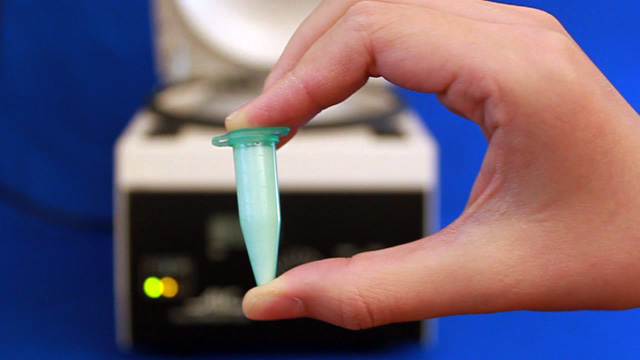
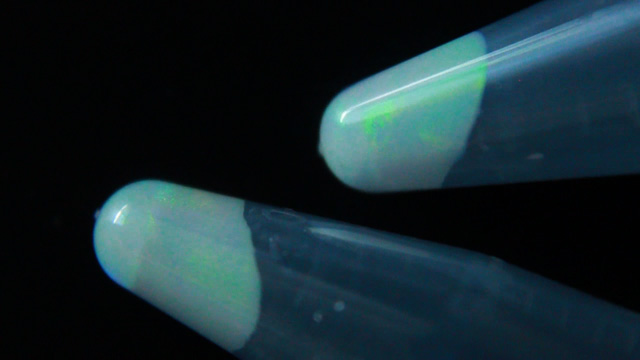
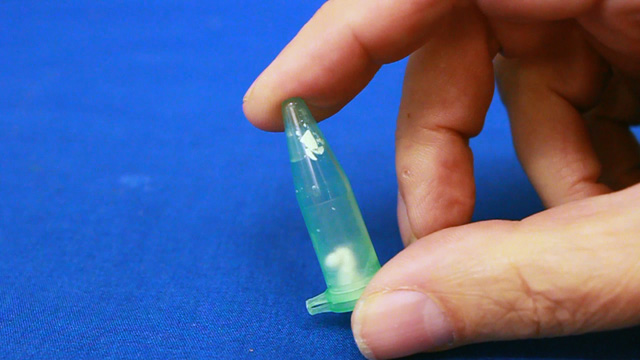
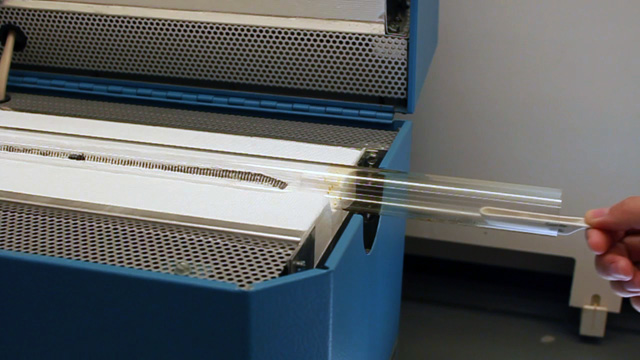
Transfer a portion of your sample to two 1.7 mL microcentrifuge tubes. Always balance the centrifuge by pairing tubes such that opposing tubes have the same amount of solution. Spin your sample at 2000 rpm for 60 minutes or longer. (Spinning at 5000 rpm does not pack as well and spinning at 10,000 rpm will crush the product.) Remove and discard the water above the polymerized polymethylmethacrylate spheres. Caution: Squeeze the dropper before immersing in the water, lower the dropper to just above the solid, and then release to remove the water. If you squeeze with the dropper in the water you will need to repeat the centrifuge step. Put the water in the organic waste. Successful product will change colors with the angle of observation or lighting; the product should look irridescent. Put the open centrifuge tube into a vacuum chamber for faster drying. Dry the sample until it freely flows in the tube. This takes 1-2 hours at 15 torr. Pour the solid into a combustion boat. Prepare a well mixed solution of 1.0 mL 100% ethanol, 1.5 mL tetraethylorthosilicate, 1.0 mL 25% hydrochloric acid. (This mixture is enough for many samples but it must be freshly prepared.) Wet the spheres with the solution but once all the spheres are wet stop adding liquid or the product may be too dense. Place the combustion boats into the quartz liner of a tube furnace (or a ventilated box furnace) in a hood. Ramp the temperature at 2 degrees C/minute from room temperature to 300 degrees C to complete the silica formation. Hold at 300 degrees C for 2 hours. Ramp the temperature at 2 degrees C/minute to 550 degrees C to decompose the polymethylmethacrylate spheres. Hold at 550 degrees C overnight (ten hours). Cool the oven to room temperature. High quality samples will be apparent by their opalescence.
SEM
Stick a portion of your sample to the carbon tape on the SEM holder and add your name to the sample map. Samples will then need to be sputtered since SiO2 is non-conducting. On our instrument (using 25kV with spotsize 27 and z 15mm) it should be possible to see holes at 3000x. Look carefully. Hole size is better measured using multiple holes in a line and then dividing by the number of holes measured. Be sure to include your analysis in your laboratory notebook.
Properties
- What color is your product in air? (The index of refraction of silica is 1.460 and of air is 1.000)
- Add a drop of ethanol to a small portion of your product. What color do you observe? (The index of refraction of ethanol is 1.360)
- Add a drop of toluene to a small portion of your product. What color do you observe? (The index of refraction of toluene is 1.496)
Calculations and Conclusions
- Summarize the evidence that you made the desired product.
- Based on your SEM data, predict the absorption wavelength for air, ethanol, and toluene. How do you need to modify the equation for closed-packed spheres for the inverse material? How well do your predictions match the observed colors? Explain.
- Polymethacrylate spheres from previous lab
- 1.7 mL microcentrifuge tubes, VWR 20170-577. The product does not self-assemble in 2.0 mL Dolphin tubes.
- microcentrifuge
- vacuum chamber and vacuum source
- microfuge tube rack that fits inside the vacuum chamber
- 100% ethanol
- 25% hydrochloric acid
- tetraethylorthosilicate, Aldrich 131903
- combustion boats, Coors 60032
- tube furnace
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified March 29, 2016 .