
Preparation of an Organic Light Emitting Diode
Modification by Jason Marmon, George Lisensky, and Wendy deProphetis from Frank G. Gao and Allen J. Bard, "Solid-State Organic Light-Emitting Diodes Based on Tris(2,2'-bipyridine)ruthenium(II) Complexes," Journal of the American Chemical Society, 122(30), 7426-7427 (2000) and Hannah Sevian, Sean Muller, Hartmut Rudmann, and Michael F. Rubner, "Using Organic Light-Emitting Electrochemical Thin-Film Devices to Teach Materials Science," Journal of Chemical Education, 81(11), 1620 (2004).
A coordination complex between a transparent tin oxide electrode and an active metal electrode produces light when an external voltage is supplied. Too thin a coating of the [Ru(bpy)3](BF4)2 polyvinylalcohol layer will give a short circuit and no light; too thick a coating will have a large electrical resistance and no light.
| Procedure | Wear eye protection |
Chemical gloves recommended |
Identify the conducting side of a piece of FTO glass by using a multimeter to measure resistance. The conducting side will have a finite resistance of 20-30 ohms. Use double-stick tape to attach FTO glass with the conductive side up to a spin-coater. Remove fingerprints from the glass. Use a cotton applicator to spread a layer of [Ru(bpy)3](BF4)2 polyvinylalcohol solution on the center of the glass. Surround with a splatter shield and spin at 2500 rpm for 10-60 seconds. Repeat for a total of 3-4 applications, trying to keep some uncoated regions at the edges. OR instead of using the preferred spin coating method in the previous step, use double-stick tape to attach FTO glass with the conductive side up to the benchtop. Use a cotton applicator to spread a very thin layer of [Ru(bpy)3](BF4)2 polyvinylalcohol solution on the glass. Evaporate using a heat gun or hair drier. Repeat for a total of 3-4 applications, trying to keep some uncoated regions at the edges. Obtain a template mask or prepare one using a piece of duct tape on aluminum foil and punching a 2/16 inch hole. Remove any remaining moisture in the film by heating for at least a minute in a hair drier. The primary reason for failure of oLEDs to light is insufficient drying of the polymer layer before adding the active metal layer. Use a cotton swab to paint through the template with liquid gallium-indium alloy to add an active metal electrode. (This eutectic mixture of 75.5% gallium and 24.5% indium is a liquid above 16.5 degrees centigrade.) Obtain a 4.5v power supply, measure its voltage, and connect the negative lead to a blunt electrode. Connect the positive lead to the FTO glass (not the [Ru(bpy)3](BF4)2 coating). Align the blunt end of the negative lead parallel to the FTO and gently touch the negative lead to the gallium-indium. In humid environments the lifetime is greatly shortened. View from under the FTO glass (left) or view in the dark (right).
Is the circuit a diode? What happens if you reverse the polarity of the applied voltage?
Conclusions
- How many layers of the [Ru(bpy)3](BF4)2 polyvinylalcohol solution did you apply? What would you recommend? Does a thin coating or a thick coating work better?
- How many gallium-indium dots did you apply? How many of them could be made to give off light? Are there any differences between those that light and those that do not light?
- How does the circuit produce light? Use an energy level diagram to illustrate your answer and include the battery.
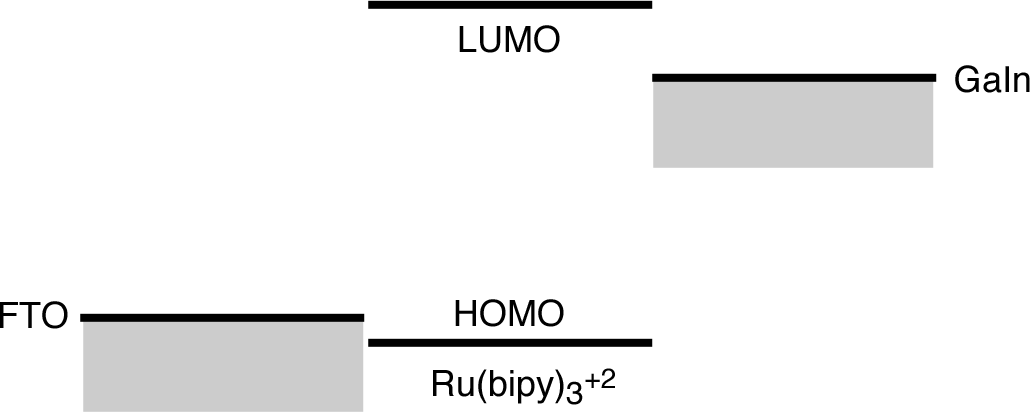
- Is the circuit a diode? How do you know? How do you distinguish your observations from what you would observe if the OLED stopped working?
- Stock Solutions for multiple preparations.
- Place approximately 0.30 g PVA (polyvinyl alcohol, Aldrich, 36,316-2, Average MW 124,000-186,000) and 10 mL of water in a 30-mL beaker. Cover the beaker with a watch glass or loosely with plastic wrap. Dissolve the PVA by heating the mixture in a microwave for 5-15 second increments. Do not allow the solution to boil. Stirring with a glass rod may help.
- Dissolve approximately 0.035 g [Ru(bpy)3](BF4)2 (Synthesis) in 3 mL of polyvinylalcohol solution. If a solution dries out, add more water and redissolve.
- GaIn Eutectic, Aldrich, 49542-5
- Equipment
- Conductive Glass (1" x 1" x 2.3mm TEC 15 glass), Hartford Glass Co, 735 E Water Street, Hartford City, IN 47348 Phone: 765-348-1282
- Ohmeter
- 2500 rpm fan and power supply, Radio Shack 273-243B 12VDC Cooling Fan, 273-1662 Universal Power Adapter
- Double-stick tape
- Template masks made from aluminum foil, duct tape, 2/16" hole punch
- Cotton swabs
- Hair dryer
- 4.5-Volt power supply
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified May 13, 2019 .











