Preparation of a Cholesteryl Ester Liquid Crystal Thermometer
Procedure by Elizabeth Boatman and George Lisensky. See "Colors in Liquid Crystals," Journal of Chemical Education, 82, 1360A (2005).
One of the principal advantages of liquid crystals is their ability to map out thermal regions of different temperature. This experiment uses a Peltier heating and cooling thermoelectric block to make small changes in temperature and an infrared thermometer to make approximate temperature measurements.Cholesteric liquid crystals contain mixtures of molecules that align in layers. Stacks of layers are rotated with respect to one another similar to DNA, spiral staircases, or screw threads. The rotation between layers increases with temperature. A color will be reflected when the pitch, the distance between layers that have the same orientation, is approximately equal to the color's wavelength of light. Since the pitch changes with temperature, the color changes with temperature.
| Procedure | Wear eye protection |
Get two pieces of clear contact paper, peel the backing off, and lay them sticky side up on a table. Obtain a vial of a cholesteryl ester mixture. Be sure to note the composition since more than one mixture will be available. Use a spatula to transfer a small amount of the gel to the sticky side of one piece of contact paper. Smear it uniformly around in the center, leaving at least a centimeter of sticky area around the edge. Cover with a second piece of contact paper, sticky sides together. Option: Packing tape also works but is more susceptible to atmospheric moisture. Trim as necessary. Rub your finger on the sandwich. Do the colors change?
Are the colors more vivid over a black background or a white background? Connect a hand crank generator to the Peltier square heating and cooling block. Turning the crank one direction will heat the block and turning the other direction will cool the block. When you are observing color changes, half a turn of the crank is a big change.
Alternatively place the sample on the outside of a beaker of hot water and watch the color changes as the beaker cools. Which color is the last color observed when heating? Which color is the last color observed when cooling? What is the sequence of colors you observe when heating or cooling?
Option: Use the infrared thermometer to record the temperature. Also record the color. Change the temperature slightly and repeat recording colors and temperatures.
This large liquid crystal film made with overhead transparency sheets is sitting on six Peltier squares that are randomly heated and cooled.
Repeat the procedure with a different composition of liquid crystal. Do you observe the same colors? Which composition has a higher melting point? How do you know?
| Color of Light | Wavelength | |
|---|---|---|
| Red | 650 nm | |
| Orange | 610 nm | |
| Yellow | 580 nm | |
| Yellow Green | 560 nm | |
| Green | 530 nm | |
| Blue Green | 500 nm | |
| Blue | 480 nm | |
| Indigo | 430 nm | |
| Violet | 410 nm | |
- Which color is the last color observed when heating? Which color is the last color observed when cooling? What is the sequence of colors you observe when heating or cooling? Do different compositions have the same color changes?
- The reflected wavelength you observe is proportional to the pitch of the liquid crystal. Why do the colors change in the observed order? Explain.
- Is the infrared thermometer reading or the liquid crystal color better at detecting small changes in temperature? Explain the reasoning for your choice.
- As the liquid crystal changes temperature it reflects different colors. Why is this better observed over a black background than over a white background?
- Which composition had a higher melting point? What happens at the molecular level that causes a melting point change as the amount of cholesteryl oleyl carbonate in your mixture is changed?
Materials
Use these materials with normal chemical precautions. Solids should not be inhaled and contact with skin, eyes, or clothing should be avoided. Wash thoroughly after handling.
- Cholesteryl Ester Liquid Crystal Mixture
- Clear contact paper
- Spatula or popsicle stick for spreading the mixture
- Scissors (optional)
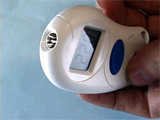
- Optional: Infrared Thermometer (http://teachersource.com)
- Genecon hand-crank generator (http://nadascientific.com)
- Peltier square with some black electrical tape on one side (Thermoelectric module 12711-5L31-03CL from http://www.customthermoelectric.com/ or check what is on clearance or at Marlow Industries or available from Amazon.
Developed in collaboration with the
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified October 10, 2018 .
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified October 10, 2018 .