|
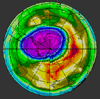 Sampling Sites |
|
|
|
|
| Ozone O3 |
|
 ppm ppm
|
|
|
| Trichlorofluoromethane CCl3F CFC-11 |
|
 ppt ppt
|
|
|
| Dichlorodifluoromethane CF2Cl2 CFC-12 |
|
 ppt ppt
|
|
|
|
Trichlorotrifluoroethane |
|
 ppt ppt
|
|
|
| Carbon tetrachloride CCl4 |
|
 ppt ppt
|
|
|
| Chloroform CHCl3 |
|
 ppt ppt
|
|
|
| Dichloromethane CH2Cl2 |
|
 ppt ppt
|
|
|
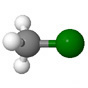
| Methyl chloride CH3Cl |
|
 ppt ppt
|
0.02 |
1.0 |
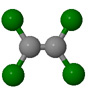
| Tetrachloroethene Cl2C=CCl2 perchloroethylene (perc) |
|
 ppt ppt
|
||
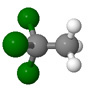
| Methyl chloroform CH3CCl3 |
|
 ppt ppt
|
|
|
|
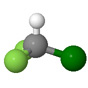
Chlorodifluoromethane |
|
 ppt ppt
|
|
|
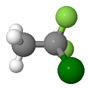
| Chlorodifluoroethane CH3CClF2 HCFC-142b |
|
 ppt ppt
|
0.07 |
17.9 |
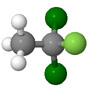
| Dichlorofluoroethane |
|
 ppt ppt
|
0.12 |
9.3 |
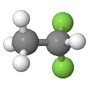
| Difluoroethane |
|
 ppt ppt
|
|
1.4 |
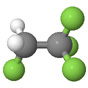
| Tetrafluoroethane |
|
 ppt ppt
|
|
14 |
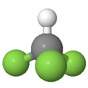
| Fluoroform |
|
 ppt ppt
|
|
270 |
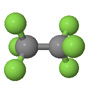
| Perfluoroethane CF3CF3 PFC-116 |
|
|
|
|
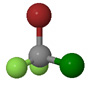
| Bromochlorodifluoromethane |
|
 ppt ppt
|
|
|
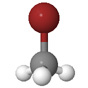
| Methylbromide |
|
 ppt ppt
|
|
|
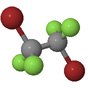
| Dibromotetrafluoroethane CBrF2CBrF2 Halon-2402 |
|
 ppt ppt
|
|
|
| Bromotrifluoromethane CBrF3 Halon-1301 |

|
 ppt ppt
|
|
|
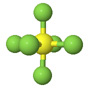
| Sulfur hexafluoride SF6 |
|
 ppt ppt
|
|
|
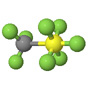
| Trifluoromethyl sulfur pentafluoride CF3SF5 |
|
 ppt ppt
|
|
|
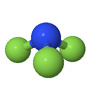
| Nitrogen trifluoride NF3 |
|
|
|
|
| Carbon tetrafluoride CF4 |
|
|
|
|
* The Ozone Depletion Potentials (ODP) is used to contrast different gases and provides a simple measure of the expected impact on ozone per unit mass emission of a gas relative to CFC-11 defined as 1.0; values are from Table 8-1, Scientific Assessment of Ozone Depletion: 2006, World Meteorological Organization (WMO) Global Ozone Research and Monitoring Program -- Report No. 50 (2007). See also US EPA Ozone-depleting Substances.
** Atmospheric lifetime is used to characterize the decay of an instanenous pulse input to the atmosphere, and represents the time the input would take to decay to 0.368 (1/e) of its original value. Values are from Table 2.14, Intergovernmental Panel on Climate Change, Climate Change 2007: The Physical Science Basis, IPCC WG1 AR4 Report. See also http://cdiac.esd.ornl.gov/pns/current_ghg.html
Ozone Depleting Molecules | Ozone Hole | ChemConnections