Microwave-assisted Synthesis of Silver Nanoparticles
The synthesis procedure shown here was adapted by Troy Dassler from Angshuman Pal, Sunil Shah, and Surekha Devi, "Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent", Materials Chemistry and Physics, 114(2-3), 530-532 (15 April 2009).
Silver nanoparticles are made by a chemical reduction of a silver salt in the presence of a stabilizing agent. Their formation can be observed by a change in color since small nanoparticles of silver are yellow. In this synthesis ethanol excited by microwaves is the reducing agent,
polyvinylpyrrolidone, 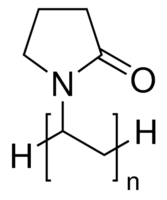 , prevents aggregation, and rapid microwave heating and agitation gives nanoparticles.
, prevents aggregation, and rapid microwave heating and agitation gives nanoparticles.
| Procedure | Wear eye protection |
Add 10 mL of 1.0% polyvinylpyrrolidone (PVP) in 100% ethanol to a small Erlenmeyer flask. Flask size is an important variable. What size did you use? Add 200 µL 0.10 M AgNO3. Cover loosely. Place the flask in the center of the microwave oven. Microwave for 5 seconds at 100% power. It may work better to set the microwave timer for a longer period and then stop the microwave after 5 seconds. The solution should be quite hot. (If not, try a different position in the oven or a different size flask.)
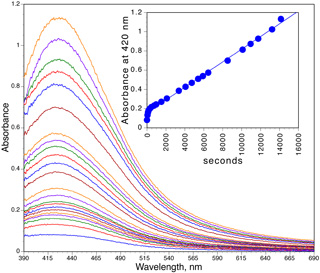 Click image for larger view
Click image for larger viewChange in absorbance with time
Record the visible absorbance spectrum.
What is the peak wavelength? What is the peak width at half the maximum height? You may need to measure the width of the longer wavelength half and then double the value to get the full width.
The presence of metal nanoparticles can be detected by their interaction with a beam of light since the oscillating electric field causes quantized light emission from the particles. Can you see a laser beam as it passes through the solution? The light from a laser pointer may be polarized with the electric field oscillation in only in one plane. Is your laser pointer polarized? If so the plasmon emission would occur only in one plane. Shine the laser through the solution and rotate the laser. What fraction of a full rotation separates the maximum and minimum observed brightness?
Optional: The solution can be used to test the antibacterial properties of silver nanoparticles.
- Stock Solutions for many batches
- 1.0% Polyvinylpyrrolidone (PVP) MW=10,000 in 100% ethanol: Dissolve 0.5 g of PVP into 50 mL of 100% ethanol. The ethanol should be anhydrous.
- 0.10 M AgNO3: Dissolve 0.17 g of AgNO3 into 10 mL distilled water. This solution should be stored in the dark.
-
Equipment
- Small Erlenmeyer flask. In some ovens a 10 mL flask works better and in other ovens a 25 or 50 mL flask works better. We have had good luck with a 10 mL flask sitting on a glass petri dish cover.
- 10 mL syringe (PVP in dry ethanol), 200 µL syringe (AgNO3 in water)
- Microwave oven (Heating for 5 seconds works for an 800W oven if the sample is positioned on a hot spot; other power ovens can be used but the heating time will need to be adjusted.)
- Laser pointer, polarizing filter
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified February 11, 2020 .