Three Syntheses of Gold Nanoparticles
The citrate synthesis procedure is from A. D. McFarland, C. L. Haynes, C. A. Mirkin, R. P. Van Duyne and H. A. Godwin, "Color My Nanoworld," J. Chem. Educ. (2004) 81, 544A. The borohydride synthesis procedure and extraction test was modified by E. Koenig and G. Lisensky from M. N. Martin, J. I. Basham, P. Chando, and S. Eah, "Charged Gold Nanoparticles in Non-Polar Solvents," Langmuir (2010) 26(10), 7410-7417. The photochemical synthesis procedure was modified by S. Rudisill and G. Lisensky from K. L. McGilvray, M. R. Decan, D, Wang and J. S. Scaiano, "Facile Photosynthesis of Unprotected Aqueous Gold Nanoparticles," J. Amer. Chem. Soc. (2006) 128, 15980-15981. The laser pointer activity was created by G. Lisensky. The electrolyte analysis of sports drinks was developed by Andrew Greenburg.
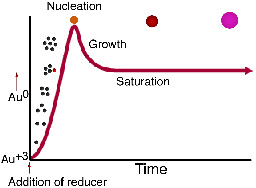
Before the addition of the reducing agent, the gold is in solution in the Au+3 form. When the reducing agent is added, gold atoms are formed in the solution, and their concentration rises rapidly until nucleation begins. The remaining dissolved gold atoms bind to the nucleation sites and growth occurs with the solution concentration of gold atoms at saturation. See "Producing gold colloids" (pdf) from IVD Technology.
Au+3 ions can be reduced to neutral gold atoms by several methods:
- Citrate ions act as a weak reducing agent and a capping agent.
The Au+ then disproportionates (reacts with itself) in water to produce Au0,
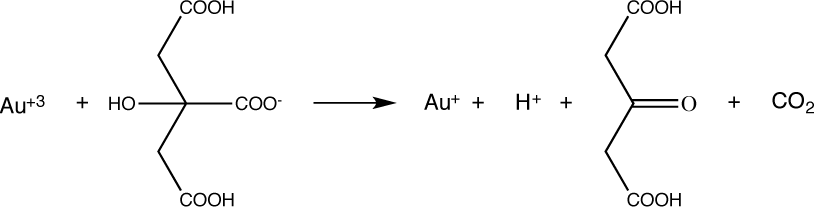
3AuCl2- → 2Au0 + AuCl4- + 2Cl-. - Borohydride acts as a strong reducing agent.
- An organic precursor reacts with light to make a ketyl radical which then reduces the Au+3 to Au+2.
2 Au+2 disproportionate (react with each other) in water to make Au+3 and Au+.
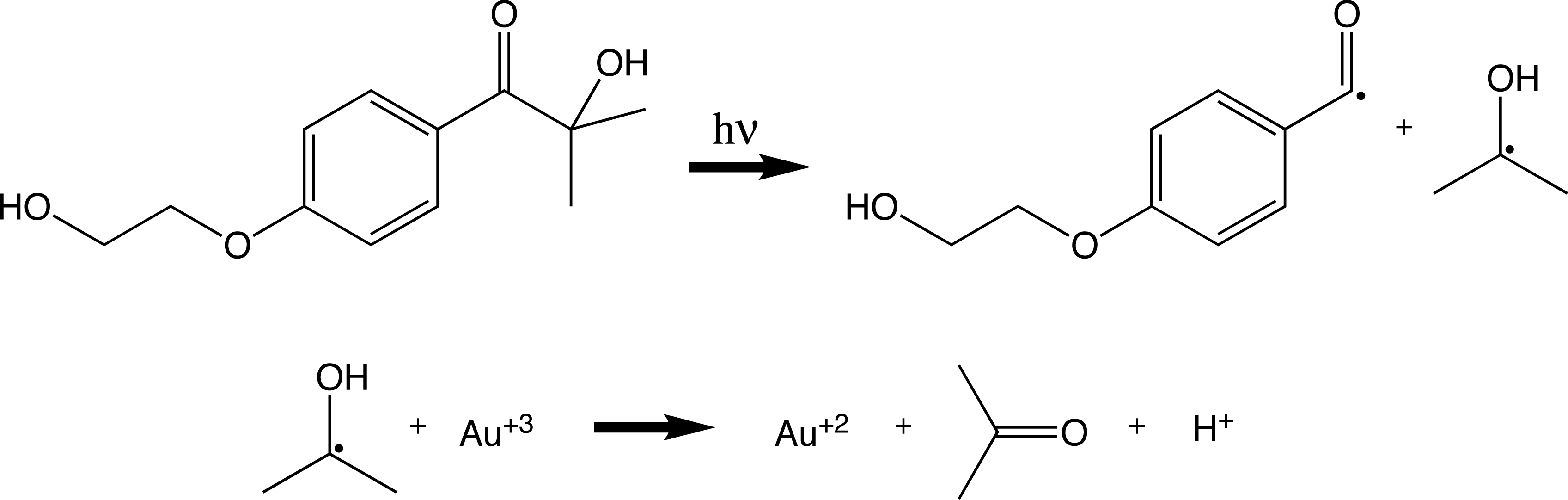
3 Au+ disproportionate to make Au+3 and 2 Au0 as in the citrate method.
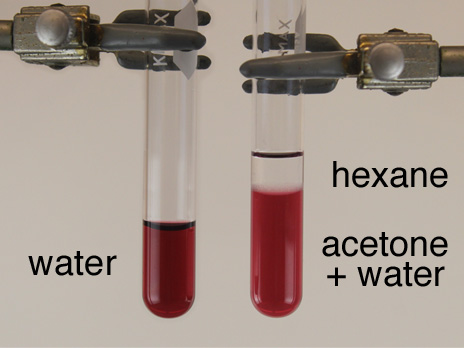
The formation of gold nanoparticles can be observed by a change in color since small nanoparticles of gold are red. The presence of this colloidal suspension can be detected by plasmon emission when the particles are excited by a laser beam. The peak width of the plasmon emission is a measure of the size distribution of the particles. Switching to a smaller anion for the capping agent allows the particles to approach more closely and a color change is observed.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Never look directly into a laser
or shine a laser at another person. Never look directly into a laser
or shine a laser at another person. |
Citrate Synthesis Method
Rinse all glassware with pure water before starting. Add 20 mL of 1.0 mM HAuCl4 to a 50 mL beaker on a stirring hot plate. Add a magnetic stir bar, bring the solution to a rolling boil, and move on to the next step. To the rapidly-stirred boiling solution, quickly add 2 mL of a 1% solution of trisodium citrate dihydrate, Na3C6H5O7.2H2O. The gold sol gradually forms as the citrate reduces the gold(III). Remove from heat when the solution has turned deep red. Ten minutes of heating for this step will be too long. (Gaps in the movie indicate equal gaps in time. The total elapsed time is approximately 10 times the movie length.)
Rinse all glassware with pure water before starting. Add 10 mL of 1.0 mM HAuCl4 to a 50 mL beaker or Erlenmeyer flask on a stirring plate. Add a magnetic stir bar and begin stirring but do not heat. When you are ready to proceed with the size test, quickly add 10 mL of 3.0 mM NaBH4 and mix. Proceed with the size test immediately. (Once you add the NaBH4 you have less than 30 seconds to do the size test for good results; please time how long it takes you and report in your notebook.)
Rinse all glassware with pure water before starting. Add 2.0 mL of 1.0 mM HAuCl4 to a 50 mL beaker. Add 4.0 mL of 1.5 mM 2-hydroxy-4'-(2-hydroxyethoxy)-2-methyl-propiophenone. Put the beaker under an illuminator designed for examing gels.
To protect yourself block the apparatus with a light shield. Illuminate with intense 300 nm light for 60-90 seconds. Record the illumination time. Never look at the UV lamps. This movie shows what is happening in the photoreactor behind the light shield. Data
Preparation before doing tests
A. The light from a laser pointer may be polarized with the electric field oscillation only in one plane. (Which of the lasers in the video of a rotating polarizer are polarized light sources?) Test laser pointers with a polarizing filter until you find one that emits polarized light. What fraction of a full rotation separates the maximum and minimum observed brightness of the transmitted beam? Be sure to record this preparation in your notebook. Tests for all methods
B. The presence of metal nanoparticles can be detected by their interaction with a beam of light since the oscillating electric field causes quantized light (plasmon) emission from the particles. Can you see a laser beam as it passes through the solution? (Would a solution dyed with red food coloring do the same thing?) C. If the light source is polarized, the plasmon emission from gold nanoparticles would occur only in one plane. Shine a polarised laser (DO NOT USE A POLARIZING FILTER!) through the solution and rotate the laser. What fraction of a full rotation separates the maximum and minimum observed brightness in the solution? Adjust the laser so the solution looks brightest from the top. What does it look like from the side? Adjust the laser so the solution looks brightest from the side. What does it look like from the top? D. Record the visible spectrum of the solution. If necessary, add additional water to the cuvette to get the absorbance on scale. What is the peak wavelength? What is the peak width at half the maximum height? You may need to measure the width of the longer wavelength half and then double the value to get the full width. E. Put a small amount of the gold nanoparticle solution in two test tubes. Use one tube as a color reference and add 5-10 drops of NaCl solution to the other tube. How does the color of the solution change as the addition of chloride makes the nanoparticles closer together? When finished put the gold solutions with chloride in the "bad gold" container.
Option: this part could be done in a cuvet with the visible spectrum recorded after each addition. Conclusions
- What is the wavelength of the visible absorption peak maximum for each of the metal nanoparticle syntheses? What is the peak width (nm) at half the maximum height (fwhm) of the visible absorption? Show your measurements on your spectra and tabulate your results for all products.
In Anal. Chem. 2007, 79, 4215-4221 it is reported that for gold nanoparticles with a diameter greater than 30 nm, the diameter equals
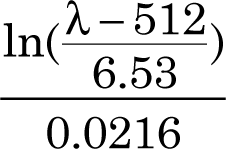 Add the calculated diameter to your table as appropriate. Add the thiol size test data to the table. In RSC Adv., 2014, 4, 3974, a series of silver nanoparticle diameters fit the equation 1.435 λ - 558.9 nm. Add the appropriate calculated diameters to your table.
Add the calculated diameter to your table as appropriate. Add the thiol size test data to the table. In RSC Adv., 2014, 4, 3974, a series of silver nanoparticle diameters fit the equation 1.435 λ - 558.9 nm. Add the appropriate calculated diameters to your table. - Which method gives the most monodispersed metal nanoparticles? How do you know?
- Why do more concentrated or stronger reducing agents tend to give more uniform nanoparticles? NaBH4 is a stronger reducing agent than Na3Citrate. The reducing strength for the photochemical method depends on the light intensity and the reducing strength in a microwave depends on the power. How would you rank the reducing power for all five methods based on your observed spectra?
- How should the results change if you did not stir the solutions during the reduction step? Do you have any evidence for this prediction?
- Explain your observations when you added salt solutions to the gold nanoparticle solutions. (The results are less clear for silver nanoparticles since any excess silver ion will result in a silver chloride precipitate.)
- Summarize the evidence that you made solid metal nanoparticles. Are the particles spherical?
Stock Solutions for 25 batches
- 1.0 mM hydrogen tetrachloroaurate: The solid is hygroscopic so purchase HAuCl4.3H2O (Aldrich 244597 or 520918) in 1.0 g quantities and use the entire bottle. Dissolve 1.0 g HAuCl4.3H2O in 250 mL distilled water to make a 10.0 mM stock solution of gold(III) ions that can be kept for years if stored in a brown bottle. Dilute 25 mL of stock to 250 mL to make the 1.0 mM concentration for this experiment.
- 1% trisodium citrate: Dissolve 0.5 g Na3C6H5O7.2H2O (sodium citrate) in 50 mL distilled water.
- 3.0 mM NaBH4: Dissolve 0.0284 g NaBH4 in 250 mL water. This solution should be made fresh before the experiment. BH4 slowly decomposes in neutral water to produce H2 gas. Solutions should not be kept overnight or tightly sealed.
- 1.5 mM radical precursor: Dissolve 0.034 g 2-hydroxy-4'-(2-hydroxyethoxy)-2-methyl-propiophenone in 100 mL distilled water. (Aldrich 410896)
- 0.2 mM dodecanethiol in hexane: 1.2 uL dodecanethiol in 25 mL hexane with glass pipet for dispensing.
- Acetone
- NaCl solution: Dissolve at least 0.5 g of NaCl in 10 mL distilled water or use a saturated solution.
- Optional sports drinks: Gatorade Ice, Powerade, Flavorless Pedialyte, Pickle Juice. Colorless or as little color as possible solutions work better. You can test electrolyte content in sports drinks by counting drops needed to change the color of 7 drops of gold nanoparticle solution.
Equipment
- 50 mL beaker (a beaker works better for the laser tests)
- Graduated cylinders: 25 mL (HAuCl4), 10 mL (Na3Citrate) and 10 mL (NaBH4)
- 1" or 1 cm stir bar. After the experiment clean stir bars with aqua regia (3:1 HCl:HNO3 with eye protection, chemical gloves, and a fume hood.)
- Stirring hotplate (Corning digital setting about 220 for citrate synthesis.)
- Transilluminator UV source, wooden 2x4 blocks to support upside down, cardboard box cover, power strip for external switching.
- 2 mL plastic pipets and 2-hydroxy-4'-(2-hydroxyethoxy)-2-methyl-propiophenone
- Laser pointer, polarizing filter
- Dropper bottles and test tubes or cuvets for NaCl tests
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified February 11, 2020 .