Memory Metal
Introduction
In 1965, the first of a series of metal alloys of nickel and titanium was produced by the Naval Ordnance Laboratory. These alloys are called Nitinol, for Nickel Titanium Naval Ordnance Laboratory. Many of the alloys have a rather remarkable property: they remember their shape. This "smart" property is the result of the substance's ability to undergo a phase change - a kind of atomic ballet in which atoms in the solid subtly shift their positions in response to a stimulus like a change in temperature or application of mechanical stress. A simple demonstation involves bending a sample, then exposing it to a source of heat like hot air or hot water. The sample recovers its original shape as its temperature is raised above the temperature corresponding to the phase change. This temperature may be tuned by varying the ratio of nickel to titanium atoms in the solid by a few percent relative to a 1:1 ratio.
How it works
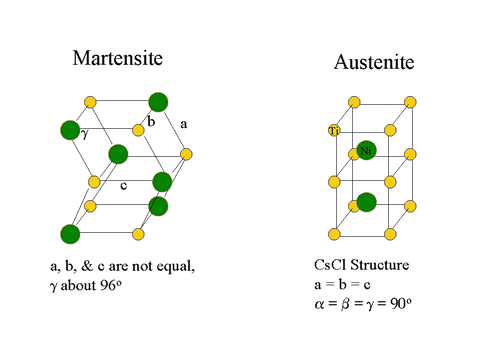
As noted above, Nitinol is an alloy of nearly equal numbers of nickel and titanium atoms, with the exact amounts varied to match the temperature of the phase change to the application. The alloy can exist in either of two structures (phases) at room temperature, depending on the exact ratio of nickel to titanium atoms. The structure found above the temperature of the phase change possesses the high symmetry of a cube and is called austenite; the structure found below the temperature of the phase change is much less symmetric and is called martensite. In the martensite phase the material is very elastic, while in the austenite phase the material is comparatively rigid.
Nitinol can be "trained" to have a new shape while in the austenite phase by deforming it into the desired shape. As it then cools to below the phase transition temperature, the material enters the martensite phase. In the martensite phase the shape can then be changed by mechanical stress: groups of atoms that were "leaning" in one direction will accommodate the mechanical stress by "leaning" in another direction, as allowed by the less symmetric structure. The sample will revert to the shape enforced upon it while it was in the austenite phase by returning it to the austenite phase through an increase in its temperature. The thermal energy acquired by the shape through heating it provides the energy the atoms need to return to their original positions and the sample to its original shape.
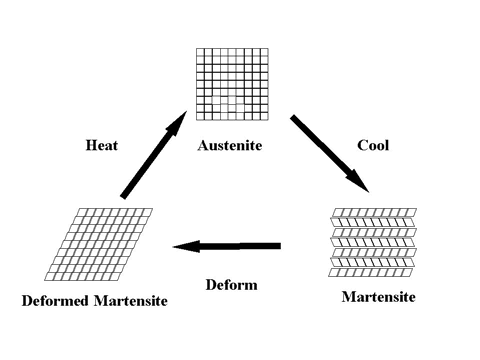
The transformation from austenite to martensite can be accomplished in 24 different ways. These 24 ways of producing martensite from austenite are the result of the symmetric CsCl structure having 6 equivalent face diagonal planes, each of which can shift in one of two directions and can distort (shear) in one of two directions, 6 x 2 x 2 = 24.
| A spring made of shape memory metal in its martensite phase. | The same spring stretched to a new shape. | In warm water or with a stream of hot air, the spring returns to its "trained" shape by heating it to above the temperature of the phase change into the more rigid austenite phase. |
Applications
Many diverse applications have been developed for "memory metal" and other "smart" materials. These uses include eyeglass frames, coffee pot thermostats , electrical connectors, deicing system, heat pipes, clamps, and sculptures.
Research is underway on using shape memory alloys to deploy solar arrays and antennae on satellites and to control the balance on helicopter rotor blades.
The biocompatibility of NiTi allows its use in many medical applications such as: vascular stents, anchors for attaching tendons to bone, medical guidewires, medical guidepins, root canal files, bendable surgical tools, and devices for closing holes in the heart.
Another important attribute of nitinol in medicine is its superelasticity.
Other shape memory materials include gold cadmium, copper-aluminum-nickel, copper-zinc-aluminum, and iron-manganese-silicon alloys.
Other Examples of Memory Metal
- Mars rover. Cumulative dust deposition is measured by rotating a quartz cover from a solar cell on the Sojourner rover (nearest corner in the photograph). Electrical heating of a 3-cm NiTi wire decreases its length by 5% to overcome a conventional return spring. Dust accumulated linearly at 0.28% per day, having implications for solar power production.

(Mars Pathfinder, July 5, 1997, http://photojournal.jpl.nasa.gov
JPL image PIA01551)
Top view schematic of the solar cell with removable cover.
- Orthodontic archwires. The archwire of these braces used in orthodontia
is made of memory metal to apply pressure
uniformly to the teeth.
- Flexible eyeglass frames. Bending the memory metal eyeglass
frames converts the metal from the rigid austenite structure
to the more flexible martensite structure. When this mechanical
stress is removed, the frames return to their original shape
and austenite structure. See this exact pair of frames distort
when exposed to liquid nitrogen.
- Radius vascular stents
Quicktime movie showing effects of SuperelasticityQuicktime movie showing effects of Phase Transformation - Toys. A company called NanoMuscle marketed a small actuator. These actuators are aimed at the toy market as reported in "Toy Story: Materials Engineering at Play".
Memory Metal Kits
A Memory Metal kit is available from the Institute for Chemical Education . This kit includes a booklet describing memory metal and a piece of memory metal wire "trained" to spell out "ICE".

| Memory metal wire can be "trained" using a jig such as is shown here. The wire is wound tightly around the peg at the left and then wound around the pins of the jig. It is then placed in an oven at 500-550oC. |

The wire can then be distorted by pulling on the ends to straighten it.

| The wire can revert back to the ICE configuration with a little heat from hot water or a hair dryer. |
Samples of shape memory metal and devices made from "smart" materials are also available from: Educational Innovations (see: Memory wire/Muscle wire).
References
Theory
Applications
- Article: 'Memory Metals Finding Their Way Into Golf Clubs, Helicopters, Operating Rooms, Bath Tubs and Factories'
- Flexon Eye Glass Frames



