

The name F-center comes from the German word for color, farbe, as crystals containing these point defects are highly colored. These defects have been investigated by various spectroscopic techniques. The F-centers are point defects and can be readily formed in alkaline halides with the aid of a source of ionizing radiation, such as a Tesla coil or X-ray source. Apparently the ionizing radiation causes the loss of electrons from halide ions. An electron then becomes trapped in the halide ion vacancy. The color is the result of the absorption of a photon by the trapped electron and excitation from the ground state to an excited state for the F-center. This is a classic case of particle in a box.
One source of ionizating radiation is a Tesla coil. Another is a source of X-ray radiation such as a powder diffractometer or synchrotron. Still another source of ionizing radiation would be a strong gamma emitter. F-centers can also be formed by heating a alkaline halide crystal and metal vapor. The color depends on the size of the defect hole that in turn depends on which halide was removed. A representation of an F-center in KCl is shown below.
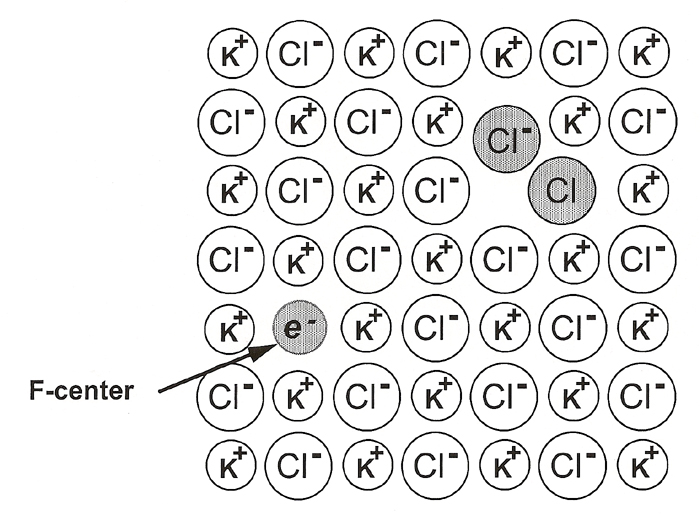 |
From Figure 6.14, page 178, Material Science Companion for Teaching General Chemistry |
| "An F-center is an electron trapped in a halide vacancy. Other defects such as the formation of Cl2- species (indicated by the shaded pair of spheres) occur for charge balance." |
Exactly how the F-center is formed, is not known. One possible mechanism involves the ionizing radiation interacting with the alkaline halide, causing a halide ion to lose an electron, producing a halogen atom. The electron then moves throughout the crystal until it finds a halide vacancy. There the electron is captured by the six surrounding alkaline metal ions. The halogen atom can then interact with a halide ion to form a molecular ion such as Cl2-.
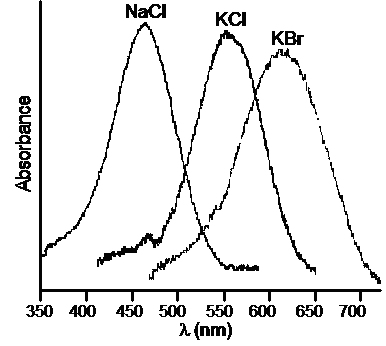 |
| From Figure 6.16, page 180, Material Science Companion for Teaching General Chemistry |
| " Absorption spectra from F-centers in NaCl, KCl, and KBr at 298 K. These spectra were obtained in air." |
The figure above shows the transmission UV-visible spectra for large crystals of NaCl, KCl, and KBr after they were irradiated with a Tesla coil while under vacuum. The color depends on the size of the defect hole that in turn depends on which halide was removed.
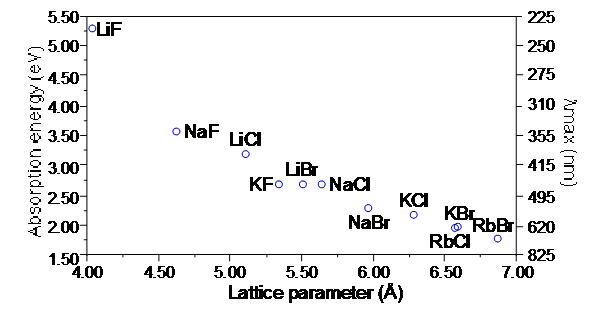 |
From Figure 6.17, page 181, Material Science Companion for Teaching General Chemistry |
| " Plot of color F-center absorption maxima as a function of the lattice parameter at 298 K." |
The plot shown above shows the relationship between the unit cell lattice parameters for these cubic materials as a functions of the energy necessary to remove an electron to form the F-center and thus the coloration. It can be shown that this energy is a function of the unit cell edge parameter, a, in the equation E ~ a-1.8.
 |
 |
In the image at the left above, a sample of KBr is being irradiated with a Tesla coil. In the right image we see crystals of NaCl, KCl, and KBr after irradiation with a Tesla coil. |
 |
The Rigaku powder X-ray diffractometer used to make the samples of F-centers seen below. |
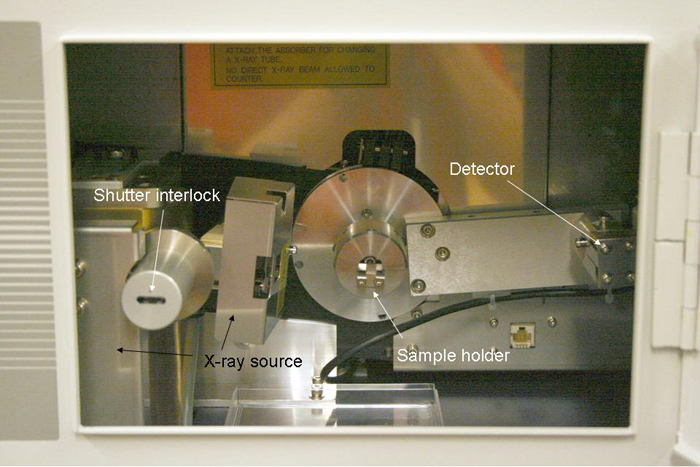 |
This view shows the X-ray source on the left, the sample holder in the center, and the detector on the right inside of the cabinet. |
The samples for radiation were prepared via the routine shown in the Nanoscale Video Lab Manual on this site, see Production of F-centers. They were then placed inside the cabinet of the diffractometer, mounted in the sample holder. The cabinet door was closed and the samples were irradiated for varying amounts of time, whatever was necessary to get a reasonable amount of color change due to the formation of the F-center defects.
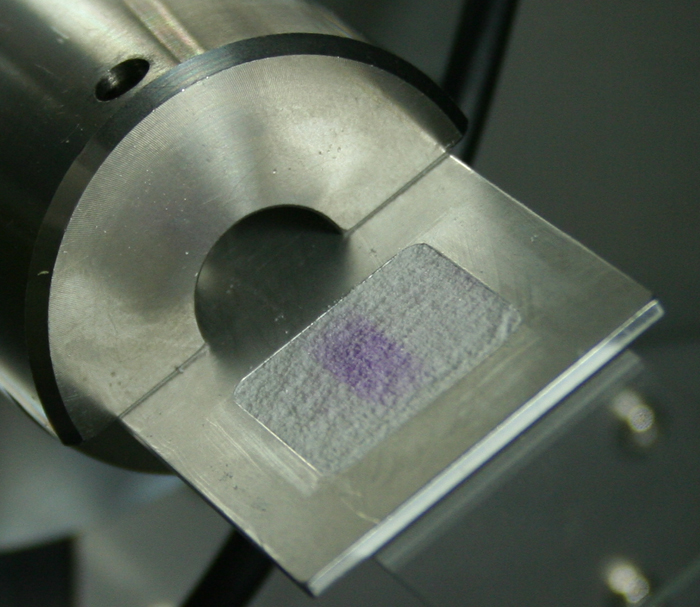 |
This sample of KCl is still mounted in the diffractometer, Note the violet color of the part of the sample that was in the beam of the X-rays. |
Each alkali halide exhibits a different color due to the F-center defect. Those samples exposed to X-rays loose their color much faster than those treated with the Tesla coil. The KBr sample decayed so fast that it had to be viewed inside the cabinet of the diffractometer.
 |
This sample of NaCl shows a golden color for the section of the sample that was in the beam of the X-rays. |
The KBr sample decayed so fast that it had to be viewed inside the cabinet of the diffractometer.
 |
This sample of KBr shows a aquamarine color for the section of the sample that was in the beam of the X-rays. |
Samples of alkaline halides which have been irradiated with gamma radiation for a long time retain their color than those samples produced by Tesla coils or X-ray radiation. Gamma irradiated NaCl is brown in color. Upon heating on a hot plate in a dark room, orange light is given off as the salt changes to its normal white color. In a lighted room, the brown color is lost upon contact with the hot plate as seen in the figure below.
 |
The NaCl on the spatula has been strongly irradiated with gamma radiation. |
A movie of the thermoluminescence of a sample of this NaCl sample is available in the F-Center page of the Cineplex or directly. The mechanism for this process is thought to involve the thermal energy of the hotplate allowing the trapped electrons of the F-center to become free and able to recombine with the electron-deficient defect Cl2- ion. The energy of the recombination is partially released as visible light. Under room lights the change of color is quite apparent. In a dark room, the material actually shows some small flashes of light during the recombination process.
 |
The left beaker has been irradiated with gamma radiation. Compare its color to that of the untreated beaker on the right. |
One proposed mechanism for the formation of F-centers involves the high energy radiation with alkaline halide, causing a halide ion to loose an electron to form a halogen atom. The electron moves through the crystal until it locates a halide vacancy.
Similar defects result in the colors of smoky quartz and amethyst which are composed predominantly SiO2. Very small amounts, a few parts per thousand, of Al3+ (smoky quartz) and Fe3+ (amethyst) are substituted for Si4+. Electrical neutrality is maintained by the placement of a proton in an interstitial site.