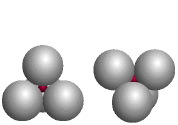
| The red atoms fill tetrahedral holes formed by four identical larger atoms; the coordination number of each red atom is four. | |
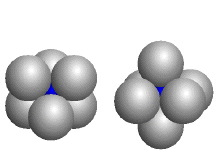
| The blue atoms fill octahedral holes formed by six identical larger atoms; the coordination number of each blue atom is six. | |
A range of radius ratios will prevent like-charged ions from touching and making the lattice unstable. |
|
Coordination | What Happens to Acid Rain? | ChemConnections