


Freshwater Acidification

Introduction
The pH of water of lakes and streams is predominantly determined by the soil and rock types of the area, since 90% of the water entering these water courses has passed through the ground. Only 10% of water in lakes and streams comes directly from precipitation.
Sweden has over 85,000 lakes that are greater than one hectare in size. Of these, 14,000 are acidified by air pollution, 4,000 being severely acidified. The number of acidified lakes would be in the order of 17,500 if liming had not been carried out to restore the pH of many of Sweden's waters over recent years. Acid sensitive species are absent from around 40% of Sweden's rivers and streams.
Acidification of a lake occurs over time. At first the natural buffering capacity of the lake neutralises the additional acidity entering the lake but at some point, the lake buffering capacity runs out and the acidity of the water increases rapidly. In time, the lake water stabilises at a certain acidity, maintaining a small number of species of plants and animals but often cannot support any fish. An example of a Swedish lake which has experienced these stages of acidification is lake Gårdsjön, near Gothenburg in the south west of the country; a lake of 31 hectares.
Lake Gårdsjön
In the late 1940s, Gårdsjön had a pH of 6.25. By the early 1960s the pH level had fallen to less than 6.0, with pH levels of 4.3-5.5 during snowmelt. By 1970, the pH had fallen to 4.5-5.2 for the whole year. Scientists have helped to identify the causes of acidification of this lake by diatom stratification. A slow natural decline in pH from 7.0 to 6.0 is thought to have occurred over many centuries before the 1950s. After 1950 rapid acidification began to occur. This coincided with a rapid increase in the number of soot particles from coal and oil burning, which were identified in the lake sediment analysis for the post 1950 period. It is also during these recent decades that pH measurements have rapidly fallen and that many species of fish have declined. These species include perch (Perca fluviatilis), roach (Leuciscus rutilus), Northern pike (Esox lucius) and European eel (Anguilla anguilla).
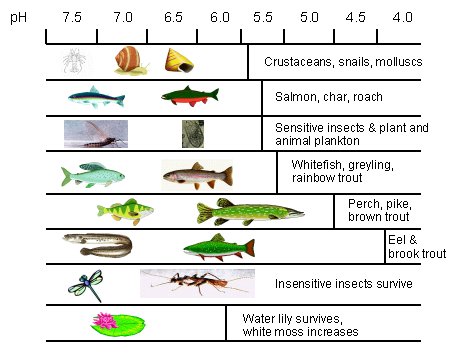
An acid lake is usually very clear, deceptively looking very beautiful but is in fact clear due to the absence of floating plankton. The light which reaches the bottom of the clear, acidified Swedish lakes enables Sphagnum moss to thrive to the extent that it often carpets the lake floor. Water lilies can also survive because their long roots can reach into sediment areas. However, species adversely affected include lobelia, quillwort and shore weeds.
Aquatic life first to be affected by lake acidification are crayfish, shrimps, snails, mussels and some species of mayfly. The fish most sensitive to a fall in pH are minnows, salmon, roach and trout, as shown in Table 4.1. Once a lake pH falls to 4.5-5.0, all that usually remains are bog moss, certain plankton species and the hardiest insects.
The leaching of nutrients from soil, released often as a result of acidification causes additional problems in lakes and water courses. Aluminium is absorbed by fish and can be stored in various organs. It can also form aluminium hydroxide on the gills, reducing blood oxygenation and causing the fish, as a result, to suffocate. Other metals such as mercury, lead, zinc and cadmium can be taken up by plants and animals causing toxicity problems. Heavy metal contamination may be passed through the food chain causing high levels of metals to be found, for example in fish, fish-eating birds and humans eating contaminated fish.
Hence, an acid lake is not a dead lake but one that maintains a poorer range of plant and animal life. This may then lead to changes in the food chain. Insect species increase as fish species are lost and this, in turn, leads to a loss of fish-eating birds and other predators.
Acidified lakes in Sweden may be restored in the short term by liming. This raises the pH of the water through the addition of powdered limestone. Each year thousands of tonnes of limestone are sprayed on Swedish lakes and watercourses, by means of trucks, boats or helicopters. The cost of liming in Sweden in 1992 was 210 million kronor. Liming causes aluminium and other metals to precipitate and fall to the bottom of the lake which may cause toxicity problems for organisms living on the lake bed. Liming provides only a temporary solution, hence it is far better to attack the source of the problem by reducing emissions of acidifying pollutants at the source.
Exercise
What causes the acidity of water entering lakes and watercourses?
How much of the water added to a lake enters directly as a result of rain or other precipitation?
What percentage of Swedish lakes are acidified?
What percentage of lakes in Sweden are severely acidified?
In your own words describe what the buffering capacity of a lake is.
When did the buffering capacity of Lake Gårdsjön run out?
What is a diatom? (Use a dictionary if necessary.)
What causes did the diatom analysis suggest for the rapid acidification process after the 1950s?
For what reason does an acid lake look clear?
Which aquatic life is affected first by lake acidification?
Give examples of aquatic life that are very sensitive to a fall in pH.
What species of aquatic life can survive in water more acid than pH 5?
How does soil acidification affect lake life?
How can an acid lake indirectly affect humans?
What good does liming do to acidified lakes?
What percentage of Swedish lakes have been limed?
How much would it cost to lime 14 000 lakes if it costs 210 million kronor to lime 4,000 lakes?
What is the best way to prevent lake acidification?